Influenza A virus subtype H5N1
 |
According to the FAO Avian Influenza Disease Emergency Situation Update, H5N1 pathogenicity is continuing to gradually rise in endemic areas but the avian influenza disease situation in farmed birds is being held in check by vaccination. Eleven outbreaks of H5N1 were reported worldwide in June 2008 in five countries (China, Egypt, Indonesia, Pakistan and Vietnam) compared to 65 outbreaks in June 2006 and 55 in June 2007. The "global HPAI situation can be said to have improved markedly in the first half of 2008 [but] cases of HPAI are still underestimated and underreported in many countries because of limitations in country disease surveillance systems".[3] On December 21, 2009 the WHO announced a total of 447 human cases which resulted in the deaths of 263.[4]
A filtered and purified Influenza A vaccine for humans is being developed and many countries have recommended it be stockpiled so if an Avian influenza pandemic starts jumping to humans,the vaccine can quickly be administered to avoid loss of life. Avian influenza is sometimes called avian flu, and commonly bird flu.
Contents[show] |
[edit] Overview
HPAI A(H5N1) is considered an avian disease, although there is some evidence of limited human-to-human transmission of the virus.[5] A risk factor for contracting the virus is handling of infected poultry, but transmission of the virus from infected birds to humans is inefficient.[6] Still, around 60% of humans known to have been infected with the current Asian strain of HPAI A(H5N1) have died from it, and H5N1 may mutate or reassort into a strain capable of efficient human-to-human transmission. In 2003, world-renowned virologist Robert G. Webster published an article titled "The world is teetering on the edge of a pandemic that could kill a large fraction of the human population" in American Scientist. He called for adequate resources to fight what he sees as a major world threat to possibly billions of lives.[7] On September 29, 2005, David Nabarro, the newly appointed Senior United Nations System Coordinator for Avian and Human Influenza, warned the world that an outbreak of avian influenza could kill anywhere between 5 million and 150 million people.[8] Experts have identified key events (creating new clades, infecting new species, spreading to new areas) marking the progression of an avian flu virus towards becoming pandemic, and many of those key events have occurred more rapidly than expected.Due to the high lethality and virulence of HPAI A(H5N1), its endemic presence, its increasingly large host reservoir, and its significant ongoing mutations, the H5N1 virus is the world's largest current pandemic threat and billions of dollars are being spent researching H5N1 and preparing for a potential influenza pandemic.[9] At least 12 companies and 17 governments are developing pre-pandemic influenza vaccines in 28 different clinical trials that, if successful, could turn a deadly pandemic infection into a nondeadly one. Full-scale production of a vaccine that could prevent any illness at all from the strain would require at least three months after the virus's emergence to begin, but it is hoped that vaccine production could increase until one billion doses were produced by one year after the initial identification of the virus.[10]
H5N1 may cause more than one influenza pandemic as it is expected to continue mutating in birds regardless of whether humans develop herd immunity to a future pandemic strain.[11] Influenza pandemics from its genetic offspring may include influenza A virus subtypes other than H5N1.[12] While genetic analysis of the H5N1 virus shows that influenza pandemics from its genetic offspring can easily be far more lethal than the Spanish Flu pandemic,[13] planning for a future influenza pandemic is based on what can be done and there is no higher Pandemic Severity Index level than a Category 5 pandemic which, roughly speaking, is any pandemic as bad as the Spanish flu or worse; and for which all intervention measures are to be used.[14]
[edit] Signs and symptoms
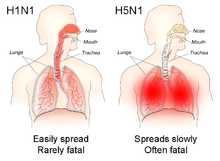
The reported mortality rate of highly pathogenic H5N1 avian influenza in a human is high; WHO data indicates that 60% of cases classified as H5N1 resulted in death. However, there is some evidence that the actual mortality rate of avian flu could be much lower, as there may be many people with milder symptoms who do not seek treatment and are not counted.[18][19]
In one case, a boy with H5N1 experienced diarrhea followed rapidly by a coma without developing respiratory or flu-like symptoms.[20] There have been studies of the levels of cytokines in humans infected by the H5N1 flu virus. Of particular concern is elevated levels of tumor necrosis factor-alpha, a protein that is associated with tissue destruction at sites of infection and increased production of other cytokines. Flu virus-induced increases in the level of cytokines is also associated with flu symptoms including fever, chills, vomiting and headache. Tissue damage associated with pathogenic flu virus infection can ultimately result in death.[7] The inflammatory cascade triggered by H5N1 has been called a 'cytokine storm' by some, because of what seems to be a positive feedback process of damage to the body resulting from immune system stimulation. H5N1 induces higher levels of cytokines than the more common flu virus types.[21]
[edit] Genetics
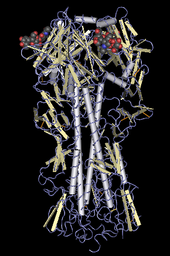
Asian lineage HPAI A(H5N1) is divided into two antigenic clades. "Clade 1 includes human and bird isolates from Vietnam, Thailand, and Cambodia and bird isolates from Laos and Malaysia. Clade 2 viruses were first identified in bird isolates from China, Indonesia, Japan, and South Korea before spreading westward to the Middle East, Europe, and Africa. The clade 2 viruses have been primarily responsible for human H5N1 infections that have occurred during late 2005 and 2006, according to WHO. Genetic analysis has identified six subclades of clade 2, three of which have a distinct geographic distribution and have been implicated in human infections: Map
- Subclade 1, Indonesia
- Subclade 2, Europe, Middle East, and Africa (called EMA)
- Subclade 3, China"[11][23][24]
[edit] Terminology
H5N1 isolates are identified like this actual HPAI A(H5N1) example, A/chicken/Nakorn-Patom/Thailand/CU-K2/04(H5N1):- A stands for the species of influenza (A, B or C).
- chicken is the species the isolate was found in
- Nakorn-Patom/Thailand is the place this specific virus was isolated
- CU-K2 identifies it from other influenza viruses isolated at the same place
- 04 represents the year 2004
- H5 stands for the fifth of several known types of the protein hemagglutinin.
- N1 stands for the first of several known types of the protein neuraminidase.
As with other avian flu viruses, H5N1 has strains called "highly pathogenic" (HP) and "low-pathogenic" (LP). Avian influenza viruses that cause HPAI are highly virulent, and mortality rates in infected flocks often approach 100%. LPAI viruses have negligible virulence, but these viruses can serve as progenitors to HPAI viruses. The current strain of H5N1 responsible for the deaths of birds across the world is an HPAI strain; all other current strains of H5N1, including a North American strain that causes no disease at all in any species, are LPAI strains. All HPAI strains identified to date have involved H5 and H7 subtypes. The distinction concerns pathogenicity in poultry, not humans. Normally a highly pathogenic avian virus is not highly pathogenic to either humans or non-poultry birds. This current deadly strain of H5N1 is unusual in being deadly to so many species, including some, like domestic cats, never previously susceptible to any influenza virus.[27]
[edit]

HA codes for hemagglutinin, an antigenic glycoprotein found on the surface of the influenza viruses and is responsible for binding the virus to the cell that is being infected. NA codes for neuraminidase, an antigenic glycosylated enzyme found on the surface of the influenza viruses. It facilitates the release of progeny viruses from infected cells.[28] The hemagglutinin (HA) and neuraminidase (NA) RNA strands specify the structure of proteins that are most medically relevant as targets for antiviral drugs and antibodies. HA and NA are also used as the basis for the naming of the different subtypes of influenza A viruses. This is where the H and N come from in H5N1.
Influenza A viruses are significant for their potential for disease and death in humans and other animals. Influenza A virus subtypes that have been confirmed in humans, in order of the number of known human pandemic deaths that they have caused, include:
- H1N1, which caused the 1918 flu pandemic ("Spanish flu") and currently is causing seasonal human flu and the 2009 flu pandemic ("swine flu")
- H2N2, which caused "Asian flu"
- H3N2, which caused "Hong Kong flu" and currently causes seasonal human flu
- H5N1, ("bird flu"), which is noted for having a strain (Asian-linage HPAI H5N1) that kills over half the humans it infects, infecting and killing species that were never known to suffer from influenza viruses before (e.g. cats), being unable to be stopped by culling all involved poultry - some think due to being endemic in wild birds, and causing billions of dollars to be spent in flu pandemic preparation and preventiveness
- H7N7, which has unusual zoonotic potential and killed one person
- H1N2, which is currently endemic in humans and pigs and causes seasonal human flu
- H9N2, which has infected three people
- H7N2, which has infected two people
- H7N3, which has infected two people
- H10N7, which has infected two people
[edit] Low pathogenic H5N1
Low pathogenic avian influenza H5N1 (LPAI H5N1) also called "North American" H5N1 commonly occurs in wild birds. In most cases, it causes minor sickness or no noticeable signs of disease in birds. It is not known to affect humans at all. The only concern about it is that it is possible for it to be transmitted to poultry and in poultry mutate into a highly pathogenic strain.- 1975 – LPAI H5N1 was detected in a wild mallard duck and a wild blue goose in Wisconsin.
- 1981 and 1985 – LPAI H5N1 was detected in ducks by the University of Minnesota conducting a sampling procedure in which sentinel ducks were monitored in cages placed in the wild for a short period of time.
- 1983 – LPAI H5N1 was detected in ring-billed gulls in Pennsylvania.
- 1986 - LPAI H5N1 was detected in a wild mallard duck in Ohio.
- 2005 - LPAI H5N1 was detected in ducks in Manitoba, Canada.
- 2008 - LPAI H5N1 was detected in ducks in New Zealand.
- 2009 - LPAI H5N1 was detected in commercial poultry in British Columbia.[29]
[edit] High mutation rate
Influenza viruses have a relatively high mutation rate that is characteristic of RNA viruses. The segmentation of its genome facilitates genetic recombination by segment reassortment in hosts infected with two different influenza viruses at the same time.[31][32] A previously uncontagious strain may then be able to pass between humans, one of several possible paths to a pandemic.The ability of various influenza strains to show species-selectivity is largely due to variation in the hemagglutinin genes. Genetic mutations in the hemagglutinin gene that cause single amino acid substitutions can significantly alter the ability of viral hemagglutinin proteins to bind to receptors on the surface of host cells. Such mutations in avian H5N1 viruses can change virus strains from being inefficient at infecting human cells to being as efficient in causing human infections as more common human influenza virus types.[33] This doesn't mean that one amino acid substitution can cause a pandemic, but it does mean that one amino acid substitution can cause an avian flu virus that is not pathogenic in humans to become pathogenic in humans.
Influenza A virus subtype H3N2 is endemic in pigs in China, and has been detected in pigs in Vietnam, increasing fears of the emergence of new variant strains. The dominant strain of annual flu virus in January 2006 was H3N2, which is now resistant to the standard antiviral drugs amantadine and rimantadine. The possibility of H5N1 and H3N2 exchanging genes through reassortment is a major concern. If a reassortment in H5N1 occurs, it might remain an H5N1 subtype, or it could shift subtypes, as H2N2 did when it evolved into the Hong Kong Flu strain of H3N2.
Both the H2N2 and H3N2 pandemic strains contained avian influenza virus RNA segments. "While the pandemic human influenza viruses of 1957 (H2N2) and 1968 (H3N2) clearly arose through reassortment between human and avian viruses, the influenza virus causing the 'Spanish flu' in 1918 appears to be entirely derived from an avian source".[22]
[edit] Prevention
There are several H5N1 vaccines for several of the avian H5N1 varieties, but the continual mutation of H5N1 renders them of limited use to date: while vaccines can sometimes provide cross-protection against related flu strains, the best protection would be from a vaccine specifically produced for any future pandemic flu virus strain. Dr. Daniel Lucey, co-director of the Biohazardous Threats and Emerging Diseases graduate program at Georgetown University has made this point, "There is no H5N1 pandemic so there can be no pandemic vaccine".[34] However, "pre-pandemic vaccines" have been created; are being refined and tested; and do have some promise both in furthering research and preparedness for the next pandemic.[35][36][37] Vaccine manufacturing companies are being encouraged to increase capacity so that if a pandemic vaccine is needed, facilities will be available for rapid production of large amounts of a vaccine specific to a new pandemic strain.[edit] Public health
| The examples and perspective in this article may not represent a worldwide view of the subject. Please improve this article and discuss the issue on the talk page. (January 2010) |
Together steps are being taken to "minimize the risk of further spread in animal populations", "reduce the risk of human infections", and "further support pandemic planning and preparedness".[38]
Ongoing detailed mutually coordinated onsite surveillance and analysis of human and animal H5N1 avian flu outbreaks are being conducted and reported by the USGS National Wildlife Health Center, the Centers for Disease Control and Prevention, the World Health Organization, the European Commission, and others.[39]
[edit] Treatment
However, WHO expert Hassan al-Bushra has said:
- "Even now, we remain unsure about Tamiflu's real effectiveness. As for a vaccine, work cannot start on it until the emergence of a new virus, and we predict it would take six to nine months to develop it. For the moment, we cannot by any means count on a potential vaccine to prevent the spread of a contagious influenza virus, whose various precedents in the past 90 years have been highly pathogenic".[42]
[edit] Epidemiology
[edit] Contagiousness
H5N1 is mainly spread by domestic poultry, both through the movements of infected birds and poultry products and through the use of infected poultry manure as fertilizer or feed. Humans with H5N1 have typically caught it from chickens, which were in turn infected by other poultry or waterfowl. Migrating waterfowl (wild ducks, geese and swans) carry H5N1, often without becoming sick.[52][53] Many species of birds and mammals can be infected with HPAI A(H5N1), but the role of animals other than poultry and waterfowl as disease-spreading hosts is unknown.[54]
According to a report by the World Health Organization, H5N1 may be spread indirectly. The report stated that the virus may sometimes stick to surfaces or get kicked up in fertilizer dust to infect people.[55]
[edit] Virulence
H5N1 has mutated into a variety of strains with differing pathogenic profiles, some pathogenic to one species but not others, some pathogenic to multiple species. Each specific known genetic variation is traceable to a virus isolate of a specific case of infection. Through antigenic drift, H5N1 has mutated into dozens of highly pathogenic varieties divided into genetic clades which are known from specific isolates, but all currently belonging to genotype Z of avian influenza virus H5N1, now the dominant genotype.[32][31] H5N1 isolates found in Hong Kong in 1997 and 2001 were not consistently transmitted efficiently among birds and did not cause significant disease in these animals. In 2002 new isolates of H5N1 were appearing within the bird population of Hong Kong. These new isolates caused acute disease, including severe neurological dysfunction and death in ducks. This was the first reported case of lethal influenza virus infection in wild aquatic birds since 1961.[56] Genotype Z emerged in 2002 through reassortment from earlier highly pathogenic genotypes of H5N1[2] that first infected birds in China in 1996, and first infected humans in Hong Kong in 1997.[31][32][57] Genotype Z is endemic in birds in Southeast Asia, has created at least two clades that can infect humans, and is spreading across the globe in bird populations. Mutations are occurring within this genotype that are increasing their pathogenicity.[58] Birds are also able to shed the virus for longer periods of time before their death, increasing the transmissibility of the virus.[edit] Transmission and host range
Because migratory birds are among the carriers of the highly pathogenic H5N1 virus, it is spreading to all parts of the world. H5N1 is different from all previously known highly pathogenic avian flu viruses in its ability to be spread by animals other than poultry.
In October 2004, researchers discovered that H5N1 is far more dangerous than was previously believed. Waterfowl were revealed to be directly spreading the highly pathogenic strain of H5N1 to chickens, crows, pigeons, and other birds, and the virus was increasing its ability to infect mammals as well. From this point on, avian flu experts increasingly referred to containment as a strategy that can delay, but not ultimately prevent, a future avian flu pandemic.
"Since 1997, studies of influenza A (H5N1) indicate that these viruses continue to evolve, with changes in antigenicity and internal gene constellations; an expanded host range in avian species and the ability to infect felids; enhanced pathogenicity in experimentally infected mice and ferrets, in which they cause systemic infections; and increased environmental stability."[59]
The New York Times, in an article on transmission of H5N1 through smuggled birds, reports Wade Hagemeijer of Wetlands International stating, "We believe it is spread by both bird migration and trade, but that trade, particularly illegal trade, is more important".[60]
On September 27, 2007 researchers reported that the H5N1 bird flu virus can also pass through a pregnant woman's placenta to infect the fetus. They also found evidence of what doctors had long suspected—that the virus not only affects the lungs, but also passes throughout the body into the gastrointestinal tract, the brain, liver, and blood cells.[61]
| Country | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Total | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cases | deaths | cases | deaths | cases | deaths | cases | deaths | cases | deaths | cases | deaths | cases | deaths | cases | deaths | cases | deaths | ||||||||||
| 8 | 5 | 63% | 8 | 5 | 63% | ||||||||||||||||||||||
| 1 | 0 | 0% | 1 | 0 | 0% | ||||||||||||||||||||||
| 4 | 4 | 100% | 2 | 2 | 100% | 1 | 1 | 100% | 1 | 0 | 0% | 1 | 0 | 0% | 1 | 1 | 100% | 10 | 8 | 80% | |||||||
| 1 | 1 | 100% | 8 | 5 | 63% | 13 | 8 | 62% | 5 | 3 | 60% | 4 | 4 | 100% | 7 | 4 | 57% | 2 | 1 | 50% | 40 | 26 | 65% | ||||
| 1 | 0 | 0% | 1 | 0 | 0% | ||||||||||||||||||||||
| 18 | 10 | 56% | 25 | 9 | 36% | 8 | 4 | 50% | 39 | 4 | 10% | 22 | 9 | 41% | 112 | 36 | 32% | ||||||||||
| 20 | 13 | 65% | 55 | 45 | 82% | 42 | 37 | 88% | 24 | 20 | 83% | 21 | 19 | 90% | 8 | 7 | 88% | 170 | 141 | 83% | |||||||
| 3 | 2 | 67% | 3 | 2 | 67% | ||||||||||||||||||||||
| 2 | 2 | 100% | 2 | 2 | 100% | ||||||||||||||||||||||
| 1 | 0 | 0% | 1 | 0 | 0% | ||||||||||||||||||||||
| 1 | 1 | 100% | 1 | 1 | 100% | ||||||||||||||||||||||
| 3 | 1 | 33% | 3 | 1 | 33% | ||||||||||||||||||||||
| 17 | 12 | 71% | 5 | 2 | 40% | 3 | 3 | 100% | 25 | 17 | 68% | ||||||||||||||||
| 12 | 4 | 33% | 12 | 4 | 33% | ||||||||||||||||||||||
| 3 | 3 | 100% | 29 | 20 | 69% | 61 | 19 | 31% | 8 | 5 | 63% | 6 | 5 | 83% | 5 | 5 | 100% | 7 | 2 | 29% | 119 | 59 | 50% | ||||
| Total | 4 | 4 | 100% | 46 | 32 | 70% | 98 | 43 | 44% | 115 | 79 | 69% | 88 | 59 | 67% | 44 | 33 | 75% | 73 | 32 | 44% | 40 | 20 | 50% | 508 | 302 | 59% |
| Source: World Health Organization Communicable Disease Surveillance & Response (CSR) | |||||||||||||||||||||||||||
[edit] Society and culture
People have reacted by buying less chicken causing poultry sales and prices to fall.[70] Many individuals have stockpiled supplies for a possible flu pandemic. International health officials and other experts have pointed out that many unknown questions still hover around the disease.[71]
Dr. David Nabarro, Chief Avian Flu Coordinator for the United Nations, and former Chief of Crisis Response for the World Health Organization has described himself as "quite scared" about H5N1's potential impact on humans. Nabarro has been accused of being alarmist before and on his first day in his role for the United Nations he proclaimed the avian flu could kill 150 million people. In an interview with the International Herald Tribune, Nabarro compares avian flu to AIDS in Africa, warning that underestimations led to inappropriate focus for research and intervention.[72]



