Dopamine
| Dopamine | |
|---|---|
 | |
 | |
| Identifiers | |
| CAS number | 51-61-6 62-31-7 (hydrochloride) |
| PubChem | 681 |
| ChemSpider | 661 |
| UNII | VTD58H1Z2X |
| SMILES | |
| InChI | |
| InChI key | |
| Properties | |
| Molecular formula | C8H11NO2 |
| Molar mass | 153.18 g/mol |
| Density | 1.26 g/cm3 |
| Melting point | 128 °C, 401 K, 262 °F |
| Boiling point | decomposes |
| Solubility in water | 60.0 g/100 ml |
| Hazards | |
| R-phrases | R36/37/38 |
| S-phrases | S26 S36 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |
Contents[show] |
[edit] History
[edit] Biochemistry
[edit] Name and family
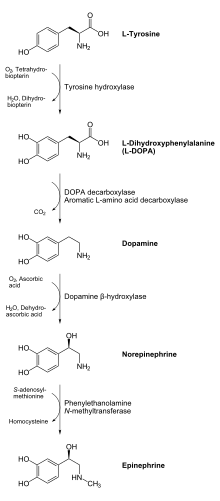
Dopamine has the chemical formula C6H3(OH)2-CH2-CH2-NH2. Its chemical name is "4-(2-aminoethyl)benzene-1,2-diol" and its abbreviation is "DA."As a member of the catecholamine family, dopamine is a precursor to norepinephrine (noradrenaline) and then epinephrine (adrenaline) in the biosynthetic pathways for these neurotransmitters.