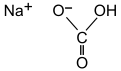
Sodium bicarbonate
Since it has long been known and is widely used, the salt has many related names such as baking soda, bread soda, cooking soda, bicarbonate of soda. Colloquially, its name is shortened to sodium bicarb, bicarb soda, or simply bicarb. The word saleratus, from Latin sal æratus meaning "aerated salt", was widely used in the 19th century for both sodium bicarbonate and potassium bicarbonate. The term has now fallen out of common usage.
Contents[hide] |
[edit] History
The ancient Egyptians used natural deposits of natron, a mixture consisting mostly of sodium carbonate decahydrate and sodium bicarbonate. The natron was used as a cleansing agent like soap.In 1791, a French chemist, Nicolas Leblanc, produced sodium carbonate, also known as soda ash. In 1846 two New York bakers, John Dwight and Austin Church, established the first factory to develop baking soda from sodium carbonate and carbon dioxide.[1]
[edit] Production
NaHCO3 may be obtained by the reaction of carbon dioxide with an aqueous solution of sodium hydroxide. The initial reaction produces sodium carbonate:
- CO2 + 2 NaOH → Na2CO3 + H2O
- Na2CO3 + CO2 + H2O → 2 NaHCO3
- Na2CO3 + CO2 + H2O → 2 NaHCO3
[edit] Mining
Naturally occurring deposits of nahcolite (NaHCO3) are found in the Eocene-age (55.8–33.9 Ma) Green River Formation, Piceance Basin in Colorado. Nahcolite was deposited as beds during periods of high evaporation in the basin. It is commercially mined using in-situ leach techniques involving dissolution of the nahcolite by heated water which is pumped through the nahcolite beds and reconstituted through a natural cooling crystallization process.[edit] Chemistry
Sodium bicarbonate is an amphoteric compound. Aqueous solutions are mildly alkaline due to the formation of carbonic acid and hydroxide ion:- HCO− 3 + H2O → H2CO3 + OH−
- NaHCO3 + HCl → NaCl + H2CO3
- H2CO3 → H2O + CO2(g)
- NaHCO3 + CH3COOH → CH3COONa + H2O + CO2(g)
- NaHCO3 + NaOH → Na2CO3 + H2O
[edit] Thermal decomposition
Above 70 °C, sodium bicarbonate gradually decomposes into sodium carbonate, water and carbon dioxide. The conversion is fast at 200 °C:[3]- 2 NaHCO3 → Na2CO3 + H2O + CO2
- Na2CO3 → Na2O + CO2
[edit] Applications
[edit] Cooking
Sodium bicarbonate was sometimes used in cooking vegetables, to make them softer, although this has gone out of fashion as most people now prefer firmer vegetables which contain more nutrients, and fibre. Bicarb destroys acids in food, including Vitamin C.
Thermal decomposition causes sodium bicarbonate alone to act as a raising agent by releasing carbon dioxide at baking temperatures. The carbon dioxide production starts at temperatures above 80 C. The mixture for cakes using this method can be allowed to stand before baking without any premature release of carbon dioxide.
[edit] Neutralization of acids and bases
Many laboratories keep a bottle of sodium bicarbonate powder within easy reach, because sodium bicarbonate is amphoteric, reacting with acids and bases. Furthermore, as it is relatively innocuous in most situations, there is no harm in using excess sodium bicarbonate. Lastly, sodium bicarbonate powder may be used to smother a small fire.[5]A wide variety of applications follows from its neutralization properties, including reducing the spread of white phosphorus from incendiary bullets inside an afflicted soldier's wounds.[6] Sodium bicarbonate can be added as a simple solution for raising the pH balance of water (increasing total alkalinity) where high levels of chlorine (2–5 ppm) are present as in swimming pools and aquariums.[7]
[edit] Medical uses
Sodium bicarbonate is used in an aqueous solution as an antacid taken orally to treat acid indigestion and heartburn.[8] It may also be used in an oral form to treat chronic forms of metabolic acidosis such as chronic renal failure and renal tubular acidosis. Sodium bicarbonate may also be useful in urinary alkalinization for the treatment of aspirin overdose and uric acid renal stones. It is used as the medicinal ingredient in gripe water for infants.[9]Bicarb has been known to be used in first aid, in treating scalding, to prevent blistering and scarring. Cover scald area with a liberal layer of bicarb and seek medical assistance.
An aqueous solution is sometimes administered intravenously for cases of acidosis, or when there are insufficient sodium or bicarbonate ions in the blood.[10] In cases of respiratory acidosis, the infused bicarbonate ion drives the carbonic acid/bicarbonate buffer of plasma to the left and, thus, raises the pH. It is for this reason that sodium bicarbonate is used in medically supervised cardiopulmonary resuscitation. Infusion of bicarbonate is indicated only when the blood pH is marked (<7.1-7.0) low.[11]
It is used as well for treatment of hyperkalemia. Since sodium bicarbonate can cause alkalosis, it is sometimes used to treat aspirin overdoses. Aspirin requires an acidic environment for proper absorption, and the basic environment diminishes aspirin absorption in the case of an overdose. Sodium bicarbonate has also been used in the treatment of tricyclic antidepressant overdose.[12] It can also be applied topically as a paste, with three parts baking soda to one part water, to relieve insect bites.[13]
Adverse reactions to the administration of sodium bicarbonate can include metabolic alkalosis, edema due to sodium overload, congestive heart failure, hyperosmolar syndrome, hypervolemic hypernatremia, and hypertension due to increased sodium. In patients who consume a high calcium or dairy-rich diet, calcium supplements, or calcium-containing antacids such as calcium carbonate (e.g., Tums), the use of sodium bicarbonate can cause milk-alkali syndrome, which can result in metastatic calcification, kidney stones, and kidney failure.
Sodium bicarbonate is also used as an ingredient in some mouthwashes. It works as a mechanical cleanser on the teeth and gums, neutralizes the production of acid in the mouth and also as an antiseptic to help prevent infections occurring.
Sodium bicarbonate can be used to cover an allergic reaction of poison ivy, oak, or sumac to relieve some of the itching that is associated with it (an alternative to buying hydrocortisone cream).[14]
Sodium bicarbonate can be used as an exfoliant. Its particles are rounded and fine in texture, making it both effective and gentle on the skin. Using baking soda as an exfoliating scrub will remove dead skin cells, which can be discolored from hyperpigmentation and scarring.
[edit] Personal hygiene
A paste made from sodium bicarbonate and a 3% hydrogen peroxide solution can be used as an alternative to commercial non-fluoride toothpastes, and sodium bicarbonate in combination with other ingredients can be used to make a dry or wet deodorant. Sodium bicarbonate is a common ingredient in alternative and natural brands of toothpaste and deodorant. It may also be used as a shampoo.[15][edit] Soda loading
Small amounts of sodium bicarbonate have been shown to be useful as a supplement for endurance athletes,[16] but overdose is a serious risk.[17][edit] As a cleaning agent
A paste from baking soda can be very effective when used in cleaning and scrubbing.[18] For cleaning aluminium objects, the use of sodium bicarbonate is discouraged as it attacks the thin unreactive protective oxide layer of this otherwise very reactive metal. A solution in warm water will remove the tarnish from silver when the silver is in contact with a piece of aluminium foil.[19]Baking soda is commonly added to the rinse cycles of washing machines (together with the detergent) as a replacement for softener and also to remove odors. Sodium bicarbonate is also effective in removing heavy tea and coffee stains from cups when diluted with warm water.
[edit] Cattle feed supplement
Sodium bicarbonate is sold as a cattle feed supplement, in particular as a buffering agent for the rumen.[edit] Miscellaneous
Sodium bicarbonate can be used to extinguish small grease or electrical fires by being thrown over the fire.[5] However, it should not be applied to fires in deep fryers as it may cause the grease to splatter.[5] Sodium bicarbonate is used in BC dry chemical fire extinguishers as an alternative to the more corrosive ammonium phosphate in ABC extinguishers. The alkali nature of sodium bicarbonate makes it the only dry chemical agent, besides Purple-K, that was used in large scale fire suppression systems installed in commercial kitchens. Because it can act as an alkali, the agent has a mild saponification effect on hot grease, which forms a smothering soapy foam. Dry chemicals have since fallen out of favor for kitchen fires as they have no cooling effect compared to the extremely effective wet chemical agents specifically designed for such hazards.[citation needed]Sodium bicarbonate is used in a process for cleaning paint called sodablasting. It can be administered to pools, spas, and garden ponds to raise pH levels.[20] It has disinfectant and antiseptic properties,[21] and it may be an effective fungicide against some organisms.[22]
Since it acts as a neutralizing agent it can be used to absorb odors which are caused due to strong acids.[citation needed] It is a tried-and-true method of used booksellers. The baking soda will absorb the musty smell, leaving the books less odorous.[23]
[edit] See also
- Carbonic acid
- Baking powder
- List of minerals
- Nahcolite
- Natron
- Natrona (disambiguation)
- Trona
[edit] References
- ^ "Company History". Church & Dwight Co.. http://www.churchdwight.com/Company/corp_history.asp.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ "Decomposition of Carbonates". General Chemistry Online. http://antoine.frostburg.edu/chem/senese/101/inorganic/faq/carbonate-decomposition.shtml.
- ^ Radiation Cookery Book 45th Edition, Radiation Group Sales Ltd 1954
- ^ a b c "Arm & Hammer Baking Soda - Basics - The Magic Of Arm & Hammer Baking Soda". Armhammer.com. http://www.armhammer.com/basics/magic/#9. Retrieved 2009-07-30.
- ^ "White Phosphorus". GlobalSecurity.org. http://www.globalsecurity.org/military/systems/munitions/wp.htm. Retrieved 2007-09-26.
- ^ "Outdoor Fun: Pool Care". Arm & Hammer Baking Soda. 2003. http://www.armhammer.com/myfamily/tips/outdoors.asp. Retrieved 2007-09-26.
- ^ "Sodium Bicarbonate". Jackson Siegelbaum Gastroenterology. 1998. http://www.gicare.com/pated/sodium_bicarbonate.htm.
- ^ List of ingredients - Life Brand Gripe Water
- ^ "Sodium Bicarbonate Intravenous Infusion". Consumer Medicine Information. Better Health Channel. 2004-07-13. http://www.betterhealth.vic.gov.au/bhcv2/bhcmed.nsf/pages/pucsodbi/$File/pucsodbi.pdf.
- ^ "Respiratory Acidosis: Treatment & Medication". emedicine. http://emedicine.medscape.com/article/301574-treatment.
- ^ Knudsen, K; Abrahamsson, J (Apr 1997). "Epinephrine and sodium bicarbonate independently and additively increase survival in experimental amitriptyline poisoning". Critical care medicine 25 (4): 669–74. doi:10.1097/00003246-199704000-00019. ISSN 0090-3493. PMID 9142034.
- ^ "Insect bites and stings: First aid". Mayo Clinic. 2008-01-15. http://www.mayoclinic.com/health/first-aid-insect-bites/fa00046.
- ^ What is Sodium Bicarbonate Used For?. Virtuowl.com. Retrieved on 2010-09-24.
- ^ Bouchard, Mallory (2010-05-04). "A Green and Healthy Beauty Secret: Going Shampoo-Free". Four Green Steps. http://www.fourgreensteps.com/infozone/featured/features/a-green-and-healthy-beauty-secret-going-shampoo-free.
- ^ Bee, Peta (2008-08-16). "Is bicarbonate of soda a performanceenhancing drug". The Times (London). http://women.timesonline.co.uk/tol/life_and_style/women/body_and_soul/article4539000.ece. Retrieved 2010-05-23.
- ^ Baking soda overdose - All Information. Umm.edu (2009-10-19). Retrieved on 2010-09-24.
- ^ "Arm & Hammer Baking Soda - Basics - The Magic Of Arm & Hammer Baking Soda". Armhammer.com. http://www.armhammer.com/basics/magic/#3. Retrieved 2009-07-30.
- ^ instructables.com
- ^ "Arm & Hammer Baking Soda - Basics - The Magic Of Arm & Hammer Baking Soda". Armhammer.com. http://www.armhammer.com/basics/magic/#8. Retrieved 2009-07-30.
- ^ Malik, Ys; Goyal, Sm (May 2006). "Virucidal efficacy of sodium bicarbonate on a food contact surface against feline calicivirus, a norovirus surrogate". International journal of food microbiology 109 (1-2): 160–3. doi:10.1016/j.ijfoodmicro.2005.08.033. ISSN 0168-1605. PMID 16540196.
- ^ Zamani, M; Sharifi, Tehrani, A; Ali, Abadi, Aa (2007). "Evaluation of antifungal activity of carbonate and bicarbonate salts alone or in combination with biocontrol agents in control of citrus green mold" (Free full text). Communications in agricultural and applied biological sciences 72 (4): 773–7. PMID 18396809.
- ^ Gail Altman (2006-05-22). "Book Repair for BookThinkers: How To Remove Odors From Books". The BookThinker (69). http://www.bookthink.com/0069/69alt.htm.
[edit] Further reading
- Bishop, D; Edge, J; Davis, C; Goodman, C (May 2004). "Induced metabolic alkalosis affects muscle metabolism and repeated-sprint ability.". Medicine and science in sports and exercise 36 (5): 807–13. ISSN 0195-9131. PMID 15126714.
[edit] External links
| Wikimedia Commons has media related to: Sodium bicarbonate |
| Wikibooks Cookbook has a recipe/module on |
| ||
| ||